VGXI’s Full Spectrum Plasmid DNA Solutions: From Discovery to Commercialization
Plasmids are small, circular, double-stranded DNA molecules that have become indispensable tools in the life sciences. They are primarily found in bacteria and are capable of self-replicating independently of chromosomal DNA. Often, the genes found in plasmids serve to confer survival advantages to bacteria, such as antibiotic resistance.
It is the combination of their stability and the ease with which they can be genetically engineered that has made them invaluable across a wide range of applications. One of the first pharmaceutical applications of plasmids was in the 1970s when researchers successfully produced human insulin via transgene-containing plasmids in Escherichia coli. Since then, advances in expression and transfection vector techniques have broadened the use of plasmids as vectors for gene cloning, recombinant protein production, and the development of advanced therapies. Today, plasmid DNA serves as a crucial raw material in the development of cell and gene therapies, and nucleic acid vaccines.
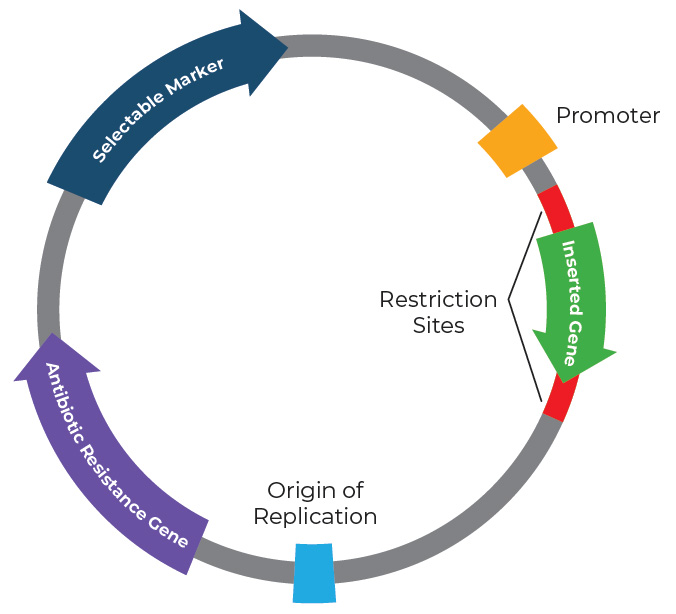
Figure 1. Map of a plasmid with several key components.
VGXI’s expertise in plasmid manufacturing, secure supply chain, and state-of-the-art facilities positions us to meet the ever-increasing global demand for plasmid DNA.
Structure of a Plasmid
Our capacity to harness and manipulate plasmids relies on the utilization of the basic components of a plasmidthat are critical to their survival and function. Let’s takea closer look at the structure of a typical plasmid, which is illustrated in Figure 1.
Origin of Replication (ORI): This is a specific DNA sequence where the replication begins.
Antibiotic Resistance Gene: Allows for selection of plasmid-containing bacterial cells.
Multiple Cloning Site (MCS): Multiple restriction enzyme recognition sites are present in the MCS, which allows for any gene of interest (GOI) to be inserted into the plasmid backbone.
Promoter: The promoter is a DNA sequence that initiates transcription, allowing RNA polymerase to bind and start the synthesis of RNA from the plasmid’s genes. Promoterscontrol the expression of genes located on the plasmid.
Gene of Interest (GOI): This is the gene that researchers want to express or study. It is inserted into the plasmid,typically within the MCS, and is under the control of the plasmid’s promoter.
Selectable Marker: In addition to antibiotic resistance genes, plasmids may include other selectable markers, such as reporter genes or fluorescent markers, to facilitate the identification of plasmid-containing cells.
Plasmids can exist in different topological forms including supercoiled, relaxed, and linear. Supercoiled plasmids are preferred for many purposes due to their stability and efficiency in gene expression and replication. Ensuring a reliable supply of high-quality supercoiled plasmid DNA is essential for a wide range of research and biotechnological endeavors.
Growing Demand for Plasmid DNA
The current demand for plasmid DNA is experiencing exponential growth, largely driven by the rapidly advancing landscape of cell and gene therapies, where many treatments are progressing through clinical development and approaching commercialization. This as further intensified during the global COVID-19 pandemic, with the development and commercialization of novel mRNA and DNA prophylactic vaccines.
Large-scale plasmid DNA production presents some complex challenges, susceptible to issues such as low yield, plasmid instability, and regulatory concerns. As a result, an increasing number of researchers and drug developers are turning to Contract Development and Manufacturing Organizations (CDMOs), such as VGXI, with advanced expertise and technology to obtain high-quality plasmid DNA that meet rigorous research and regulatory standards. As the demand for plasmid DNA continues to rise, the need for reliable manufacturing partners becomes ever more critical.
With 20 years of experience, VGXI has mastered plasmid DNA production, having developed a proprietary and scalable platform to manufacture DNA suited for any phase of the drug development cycle, from fundamental research to commercialization. VGXI’s expertise in plasmid manufacturing, secure supply chain, and state-of-the-art facilities positions us to meet the ever-increasing global demand for plasmid DNA.
VGXI’s track record includes successful delivery of high-quality cGMP plasmids for clinical trial testing of leading DNA vaccine candidates. And for personalized immunotherapy applications on a smaller scale, we’ve pioneered an accelerated manufacturing process, able to deliver cGMP plasmid DNA much faster than industry standards without compromising quality.
VGXI Plasmid Solutions
VGXI’s comprehensive range of plasmid DNA service levels caters to projects of any size, and we excel in creating customized solutions to address challenging plasmids and unique project demands. By adopting a phase-appropriate production strategy and implementing fit-for-purpose quality management, we ensure that the plasmid DNA materials we provide are not only of the highest quality but also aligned with specific requirements and timelines.
VGXI offers three distinct service categories:
Pre-Clinical Service
High purity plasmid preparations for early-stage research, in vivo animal studies, and preclinical assessments for advanced therapy development.
HD Service
A cost-effective and more rapid alternative to fully cGMP plasmid DNA, these “GMP-like” plasmids are sustainable for use as an ancillary raw material in downstream cGMP manufacturing and IND enabling studies.
GMP Service
Injectable-grade plasmid DNA with the hightest level of compliance for use in human clinical trials, diagnostic reagents, and clinical through commercial supply of raw materials for GMP cell and gene therapy production.
Our pre-clinical and HD services operate on the same platform manufacturing technology as VGXI’s cGMP Production Service, ensuring consistent quality and scalability across our entire service spectrum. This translates into the delivery of plasmid products characterized by high supercoil content and low endotoxin levels that meet phase-appropriate regulatory requirements. With plasmid concentrations ranging from 1 to 15mg/mL and fermentation scales reaching up to 100L for pre-clinical and HD services, and up to 1500L for our cGMP plasmids, you can rely on our expertise and manufacturing capabilities to drive your programs forward. At VGXI, we’re committed to excellence at every step to ensure the success of your innovations on the global stage.
For more information on our plasmid services or to request a quote, please contact us at [email protected].


