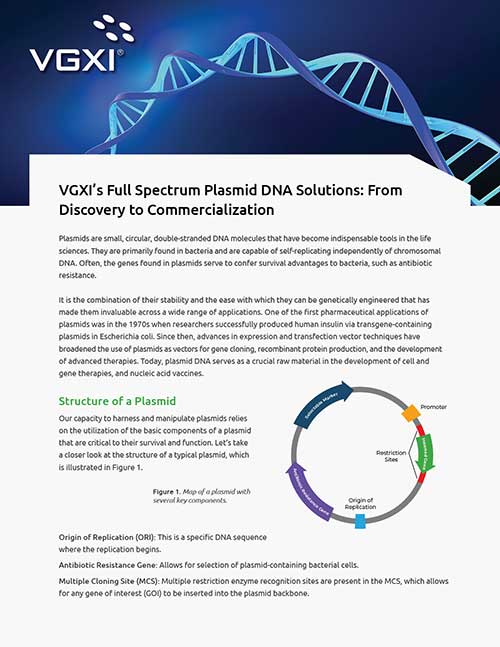
Plasmid DNA Manufacturing
At VGXI, we lay a solid foundation for your drug development program with our industry-leading DNA plasmid manufacturing and production services. We confidently support your therapeutic program with a range of pDNA service levels, scaled to meet projects of any size, at every stage. Our Plasmid DNA expertise and services are tailored to meet your phase-appropriate requirements, ensuring a seamless experience no matter where you are in the development process. We also provide customizable manufacturing options to create personalized solutions that precisely match your unique requirements and objectives.
A typical plasmid DNA manufacturing run at VGXI can span from 4 to 8 months depending on the specific requirements of each client’s project. Key steps of the VGXI manufacturing process are outlined below.
Plasmid Evaluation
Each new plasmid project brought to VGXI undergoes thorough evaluation in a small-scale production run known as Plasmid Evaluation. This pre-clinical simulation mirrors conditions of the full-scale GMP run, allowing our Process Development team to assess the plasmid’s quality and yield accurately.
Cell Banking
If required as part of the project scope, VGXI can generate a GMP Master Cell Bank in a dedicated area, starting from the fully evaluated research cell bank.
Fermentation
Our fermentation experts can optimize the process for each distinct plasmid, according to specific project requirements. The advantages of VGXI Fermentation include:
- Fully automated process
- E. coli fermentation scales up to 1500L
- Capabilities for both batch and fed-batch fermentation
- Both antibiotic and antibiotic-free selection protocols available
The fermentation process is executed under precisely controlled growth conditions and is closely monitored from the initial inoculation to the final harvest.
Lysis
Cell lysis and efficient neutralization are highly critical steps yet difficult to get right as the production scale increases. VGXI’s patented AIRMIX® Technology is a fully automated, animal-component free, and scalable process that utilizes a low-shear continuous capture method to effectively lyses cells without damaging cellular contents. This minimizes genomic DNA contamination while preserving plasmid DNA supercoiling.
Purification
We optimize downstream chromatography steps for each plasmid, to ensure effective removal of unwanted impurities like host cell genomic DNA, RNA, proteins, and endotoxin.
Concentration & Aseptic Filtration
Tangential flow ultrafiltration/diafiltration (UF/DF) is used for final concentration and buffer exchange. With precise control of transmembrane pressures (TMP), we ensure the optimal conditions to successfully achieve plasmid concentrations of up to 15mg/mL, all while preserving DNA integrity. We can formulate plasmid DNA product in a wide range of buffers and excipients to meet diverse project requirements.